A groundbreaking clinical trial has revealed that an experimental anti-amyloid drug may significantly reduce the risk of developing Alzheimer’s-related dementia in individuals genetically predisposed to the disease. The study, led by the Knight Family Dominantly Inherited Alzheimer Network-Trials Unit (DIAN-TU) at Washington University School of Medicine in St. Louis, marks a major step forward in the prevention of Alzheimer’s symptoms before they begin.
For the first time in a clinical setting, researchers have found that removing amyloid plaques from the brain years before symptoms emerge can delay the onset of Alzheimer’s dementia. These findings open new doors for preventive treatments and provide hope that delaying the disease for decades—or even preventing it altogether—may be possible.
Contents
A Closer Look at the Study

The international clinical trial involved 73 participants, all of whom carried rare genetic mutations that cause an overproduction of amyloid in the brain. This buildup all but guarantees that these individuals will develop Alzheimer’s disease in middle age. Among the participants, 22 individuals who received the drug the longest—an average of eight years—saw their risk of developing symptoms cut in half.
Dr. Randall J. Bateman, the lead researcher and professor of neurology at Washington University, emphasized the significance of these results. “Everyone in this study was destined to develop Alzheimer’s disease, yet some of them haven’t yet,” he stated. “We don’t yet know how long they will remain symptom-free—maybe a few years or maybe decades.”
The Role of Amyloid in Alzheimer’s Disease
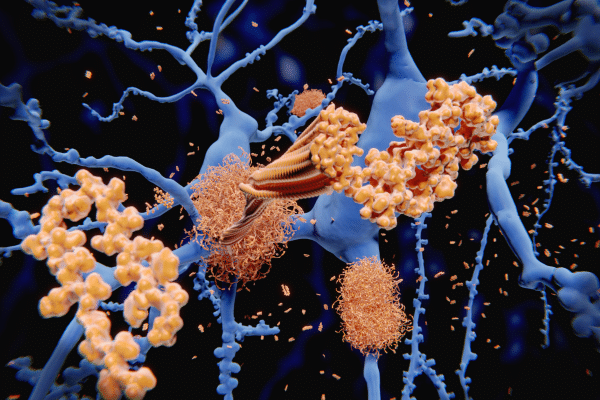
Alzheimer’s disease has long been associated with amyloid plaques—sticky protein clumps that accumulate in the brain and are thought to contribute to cognitive decline. The amyloid hypothesis suggests that blocking the formation of these plaques or removing them early enough could stop the disease before symptoms arise.
This study is the first to provide clinical trial evidence supporting that theory. Researchers focused on whether an anti-amyloid drug could prevent the onset of dementia rather than just slow it down once symptoms appear.
A Decade-Long Investigation
The participants originally enrolled in the Knight Family DIAN-TU-001 study, the first Alzheimer’s prevention trial in the world. Launched in 2012, it aimed to evaluate whether anti-amyloid drugs could act as preventive therapies.
At the conclusion of the original trial in 2020, researchers found that gantenerumab, an anti-amyloid drug developed by Roche and Genentech, reduced amyloid levels in the brain and improved some measures of Alzheimer’s-related proteins. However, cognitive benefits were not immediately observed, as many of the participants had not yet reached the stage where symptoms would be expected.
To further explore gantenerumab’s long-term effects, the study leaders initiated an open-label extension, allowing all participants with high-risk genetic mutations to continue receiving the drug, regardless of their original trial group.
Key Findings and Implications
The most significant discovery was that in the subgroup of participants who received the drug for eight years, the risk of developing Alzheimer’s symptoms was reduced by approximately 50%. This calculation factored in not only how many people developed symptoms but also the timing of their symptom onset compared to their expected age.
For participants who only received gantenerumab for two to three years in the extension study, no clear cognitive benefits were observed yet. This suggests that longer treatment durations may be necessary for true prevention.
Challenges and Limitations
While the findings are encouraging, the study had limitations. The extension phase did not include an internal control group, meaning researchers had to compare results to an external observational study. Additionally, statistical significance was only reached in the group that received the longest treatment, indicating that longer-term studies are needed to confirm the findings.
Another concern is the side effects associated with anti-amyloid drugs. Gantenerumab, like other drugs in this class, has been linked to amyloid-related imaging abnormalities (ARIA), which can cause minor brain swelling or tiny brain bleeds. These abnormalities were seen in 30% of participants in the extension phase, an increase from the original trial’s 19% due to the higher doses used. In most cases, ARIA did not cause symptoms, but two participants experienced severe cases that required them to stop taking the drug. Both individuals recovered fully.
The Future of Alzheimer’s Prevention
Despite the promising findings, gantenerumab is no longer in development. Roche and Genentech discontinued the drug in 2022 after its larger Phase 3 trials in symptomatic Alzheimer’s patients failed to show a significant slowing of clinical decline. However, researchers are not giving up.
The Knight Family DIAN-TU has launched a new trial, the Amyloid Removal Trial, to determine how long Alzheimer’s symptoms can be delayed by removing amyloid plaques. Many participants in the extension study have now switched to lecanemab, an FDA-approved anti-amyloid drug shown to slow cognitive decline in patients who already have symptoms.
Funding remains a critical factor in continuing this research. A grant proposal submitted to the National Institutes of Health (NIH) is still awaiting approval, and the Alzheimer’s Association has stepped in to provide initial funding for continued investigations.
What This Means for the General Population
While this trial focused on individuals with rare genetic mutations leading to early-onset Alzheimer’s, the results could have significant implications for the broader population. Researchers point out that both early-onset and late-onset Alzheimer’s begin with amyloid accumulation in the brain decades before symptoms appear. If similar trials in late-onset cases yield comparable results, anti-amyloid treatments could eventually become widely available for preventing Alzheimer’s disease before it starts.
Dr. Bateman remains optimistic about the future of Alzheimer’s prevention. “If late-onset Alzheimer’s prevention trials have similar results, we could soon have preventative treatments available for millions of people,” he said.
Final Thoughts
The study’s findings represent a major step forward in Alzheimer’s research, providing the first clinical evidence that removing amyloid plaques early can delay or even prevent dementia. Although gantenerumab will not be moving forward, other anti-amyloid drugs are being explored as potential preventive treatments.
With continued research, investment, and long-term studies, the dream of preventing Alzheimer’s before symptoms ever appear may soon become a reality.
Stay Updated with Breaking News
Get real-time updates on breaking stories, trending topics, and the latest headlines. Follow Dumbed Down News on X (formerly Twitter) for fast, no-nonsense coverage!
Click here to follow now: Dumbed Down News


